Project Management
/Our considerable practical and leadership experience means we are the ideal partner to assist in running your key projects, assignments and work streams.
Our inherent solutions focus and customer centric outlook allows quick identification of road-blocks. Effective solutions to keep projects on track and ensure customer needs are met.
We are open-minded, positive and flexible in the face of short-term challenges but always with one eye on the bigger picture and the longer-term view.
Verification & Validation
Ensuring devices meet regulatory standards and are safe to use or that drug manufacturing processes are controlled and effective are non-negotiable in the Healthcare and Pharma space. Marble can support you in generating all the necessary documentation V&V reports, approved DMR and Design History Files (DHF), etc. required for successful regulatory submission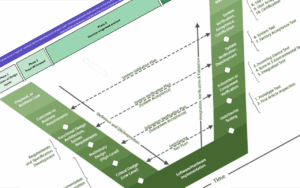
Quality Management
Marble have held ISO9001 and ISO13485 certification since 2017. Our QMS reflects the needs of an agile design and development company focusing on the quality of our work and client satisfaction. Our QMS is flexible in enabling us to work with the right controls in place for a range of clients and industries, or to seamlessly work to a client’s quality or regulatory requirements.
Project Management
At Marble we combine the core Project Management basics of Planning & Scheduling, Risk Management, and Budget Management, with specific New Product Development skills including deep technical understanding, Design for Manufacturing (DFM), stage-gate process and Regulatory & Compliance awareness. We blend these skills with motivational team management, coordinating cross-functional teams, resolving conflicts and driving teams towards shared goals.
Risk Management
Regardless of the user risk, product risk, manufacturing risk, we understand the overall project risks and goals. Enabling us to prioritise, mitigate and manage the development process to achieve success.
Tech Documentation & Specifications
We formulate, scope, write and review a range of technical documentation for our clients. Whether to support our own design work or as an independent resource for a client’s project, we have a deep understanding of the requirements to produce useful, meaningful documentation.